An examination of cranial electrotherapy stimulation (CES) on alpha-amylase levels, cortisol levels and state-trait anxiety scores in the chronically mentally ill
Strentzsch, Julie A. An examination of cranial electrotherapy stimulation (CES) on alpha-amylase levels, cortisol levels and state-trait anxiety scores in the chronically mentally ill. Doctoral Dissertation, Saint Mary’s University, San Antonio, Texas, 2008.
Device
Alpha-Stim®
Key Variable
State Anxiety
Objective
To evaluate the effect of a specified treatment course with CES on chronically mentally ill patients’ anxiety when compared to sham treatment under the same experimental conditions in subjects meeting the inclusion and exclusion criteria.
Design
This was an IRB approved 3 week randomized, sham controlled, double-blind, clinical trial.
Primary Effectiveness Endpoint
The primary effectiveness endpoint was the change from baseline in the last post-treatment scores on the State Anxiety Inventory (SAI) compared to the sham group at the end point of the study.
Secondary Outcome Measures
Secondary outcome measures of trait anxiety, cortisol and alpha-amylase levels were measured at the end of the 3 week study.
Key Inclusion Criteria
• Chronically mentally ill outpatients attending a partial hospitalization program.
• Diagnosis of anxiety by subject’s physician.
Key Exclusion Criteria
• Pregnancy.
• Presence of implanted pacemakers, pumps or stimulators.
Protocol Summary
Baseline measures were taken on the day the subjects were accepted into the study. The active and sham groups had CES treatments every day at 11 am during a therapy session. The control group attended the therapy session but was not connected to a CES device. At the end of 3 weeks, primary and secondary outcome measures were repeated.
Device Application Protocol
The active CES device was pre-set and locked by the manufacturer at 100 µA which is a subsensory level. The sham CES device was pre-set and locked by the manufacturer so that it did not emit electricity. The length of treatment, 60 minutes, was also pre-set and locked by the manufacturer for both active and sham devices. There were an equal number of active and sham devices. Randomization of the devices were done at the factory and the devices were packed in the order that they would be assigned to subjects.
Study Blinding
The subjects, investigators and staff were all masked as to the identity of the device.
Outcome Measures
The Spielberger State/Trait Anxiety Inventory was used to measure anxiety. It has established reliability and validity (American Psychological Association, 2014; Spielberger 2010).
Results
Subjects
A total of 45 subjects were enrolled and 38 subjects completed all post-test requirements; active CES group (N=15), sham group (N=15), and usual care control group (N=8). Diagnoses included bipolar disorder (N=13, 31%), generalized anxiety disorder (N=5, 12%), major depressive disorder (N=4, 10%), schizoaffective disorder (N=9, 21%) and schizophrenia (N=11, 26%).
Baseline Measurements: Group Equivalence
There were no statistically significant differences at baseline among the groups for any of the outcome measures.
Data Analysis
Data were analyzed using paired samples t-tests.
Anxiety
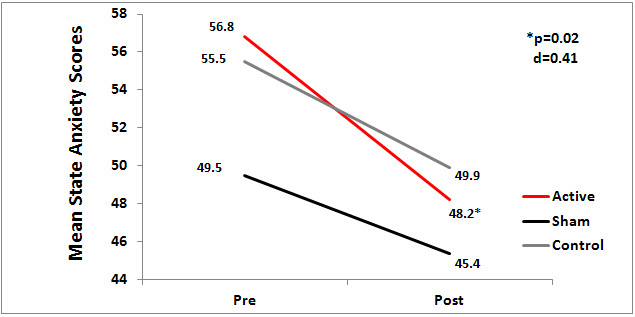
The active CES group had significantly lower scores on the State Anxiety Index (SAI), indicating less state anxiety, than the sham group (P=.02, d = -.41) or control group. As expected, since trait (chronic) anxiety is usually a stable personality trait it is less responsive to change than state anxiety (Spielberger, 2010) there was no significant difference between the active and sham groups on trait anxiety. There was no significant difference among groups on the variables of cortisol and alpha-amylase levels. Figure 1 shows results of statistical analyses of outcome measures for state anxiety for the 3 groups.
Figure 1. Mean state anxiety scores by group.
Quality of the Research
Strengths of this study are: use of a randomized, sham controlled, double-blind design (The investigator chose to use the Alpha-Stim RCT research protocol for the study); active and sham Alpha-Stim devices were pre-set and locked at the designated levels for each specific group for current level and time of treatment by the manufacturer at the factory; sham units were the same as active units, except they did not emit electricity; randomization of devices was done by the manufacturer and followed according to the protocol by the investigator; diagnosis of anxiety was verified using the criteria from the DSM-IV before subjects could be in the study; and the use of a structured and detailed protocol for the CES treatments for both active and sham groups. Previous effect sizes from other CES studies for anxiety indicate the N of the study had sufficient power to detect differences. A limitation of this 2008 study is the use of paired samples t-tests which was a common practice at that time. Today, an analysis of covariance would most likely be used and it would provide a more comprehensive description of the data. The effect size in this study for anxiety approached moderate (d=0.41) in contrast to the moderate to very large effect sizes for anxiety in other Alpha-Stim CES RCT studies. This is probably due to the limited treatment time of 3 weeks in this chronically mentally ill patient population. The significant finding of this study that CES decreases anxiety is consistent with other Alpha-Stim studies that had similar findings.
References
American Psychological Association. The State-Trait Anxiety Inventory (STAI). Accessed 4/19/2014, http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/trait-state.aspx.
Spielberger, C. D. State-Trait Anxiety Inventory. Corsini Encyclopedia of Psychology. Published Online: January 2010, http://onlinelibrary.wiley.com/book/10.1002/9780470479216.