Safety and effectiveness of cranial electrotherapy stimulation in treating children with emotional disorders
Lu XY, Wang AH, Li Y, Zhang JS, Liu BX. Safety and effectiveness of cranial electrotherapy stimulation in treating children with emotional disorders. Chinese Journal of Clinical Rehabilitation. 2005; 9(8):96-7. Download article in English. Download article in Chinese.
Device
Alpha-Stim®
Key Variables
Anxiety, Depression
Objective
The purpose of this 3 week open label study was to evaluate the safety and effectiveness of CES for the treatment of children with mixed anxiety and depression disorder (MAD).
Design
This study was an open label clinical study that included 32 children who participated in a CES course of treatment.
Primary Effectiveness Endpoints
The primary effectiveness endpoints were the change from baseline in the last post-treatment scores on the Zung Self-rating Anxiety Scale (SAS) and the Zung Self-rating Depression Scale (SDS) after 3 – 15 days of treatment.
Key Inclusion Criteria
• Male and female children, 9 – 17 years old, with mixed anxiety and depression.
• Diagnosis of MAD was done by a physician with a specialty in child psychosis using the Chinese Classification of Mental Disorders 3 (CCMD-3) criteria, after consultation and evaluation by a psychologist.
Key Exclusion Criteria
• Pregnancy, planning to become pregnant or nursing.
• Presence of implanted pacemakers, pumps or electrical stimulators.
• Use of any anxiety or depression drug therapy or participating in psychotherapy.
• Anxiety or depression state caused by schizophrenia or other physical diseases.
Protocol Summary
The current level of the Alpha-Stim CES device was adjusted to a comfortable level for each subject, between 200 to 600 µA, and the frequency was set at 0.5 Hz. Baseline measures were done prior to the first CES treatment. A course of treatment was defined as daily 20 minute CES treatments for 5 days. Endpoint measures were done after the final CES treatment.
Device Application Protocol
The length of treatment, 20 minutes, was also pre-set on the device.
Study Blinding
The “performers, evaluators and data statisticians” were blinded regarding the type of treatment that subjects were receiving.
Pre-Specified Criteria for Success
Significance was set at p<0.05.
Outcomes Measures
The Zung Self-Rating Anxiety Scale (SAS) and the Zung Self-Rating Depression Scale (SDS) were used to measure anxiety and depression. Both scale have established reliability and validity (Zung et al., 1971; 1965). Physiological measures, systolic blood pressure and pulse rate, of anxiety were also measured to validate anxiety.
Results
Subjects
Thirty-two (32) children participated in the study; 15 males and 17 females. Ages ranged from 9 to 17 years with a mean of 13 years old.
Data Analysis
Data were analyzed using t-tests and descriptive statistics.
Anxiety
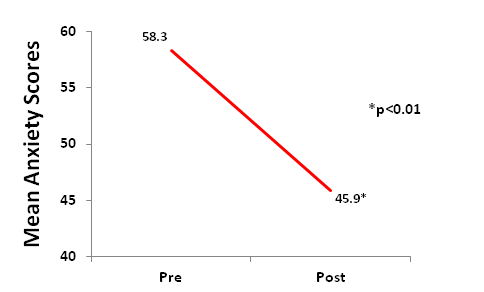
Compared to baseline scores, the SAS standard score of all subjects significantly decreased and returned to a normal value (<50) after the treatment (p<0.01), Figure 1.
Figure 1. Subjects had a significant decrease (p<0.01) in anxiety scores from baseline after CES treatments.
Depression.
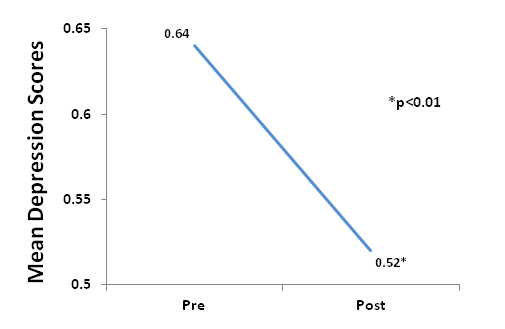
Compared to baseline scores, there was a significant decrease in SDS scores at endpoint of the study (p<0.01), Figure 2.
Figure 2. Subjects had a significant decrease in depression scores from baseline to endpoint of study (P<0.01).
Efficacy of CES
Among the 32 children, the shortest period of treatment was 3 days and the longest was 15 days with a mean of 7 days. Investigators categorized the results as significantly effective: good self-feelings, stable emotions, and good social functions, the SAS and SDS scores recovered to normal values (SAS <50, SDS <0.5). Effective: Self-feelings, emotions social functions improved some, SAS and SDS scores decreased, but did not recover to normal values; Ineffective: No improvement in self-feelings and SAS and SDS test scores did not decrease.
The total effectiveness rate was 94%; Significantly effective – 40.62%, Effective – 53.12%, Ineffective – 0.062%.
Physiological indices before and after CES treatment
Skin temperature increased significantly after treatment from baseline (p<0.01). There was a significant decrease in systolic blood pressure and pulse rate (p<0.05). These significant changes occurred in 75% of all subjects.
Adverse Effects
Three subjects occasionally felt dizziness and experienced local irritation at the electrode site. There were no serious adverse events.
Quality of the Research
This is a good clinical study. Strengths of the study are; establishing the diagnosis of MAD using specific criteria prior to inclusion in the study, use of a single-blind method, use of a pre-specified criteria for success, the operational definition for efficacy of CES using the SAS and SDS scores, and blinding of the “performers, evaluators and data statisticians” regarding the type of treatment that subjects were receiving. Limitations are; variation in the number of CES treatments for subjects, and the small sample size. While it is expected that children may miss a CES treatment, some children had more than the number of CES treatments (5) in the protocol.. The positive findings of this study are consistent with other studies on anxiety and depression that used Alpha-Stim® CES technology; CES significantly decreases anxiety and depression.
Author Affiliations
LX-Y, WA-H, LY, ZJ-S, Neurorehabilitation Center, Affiliated Beijing Children’s Hospital, Capital University of Medical Sciences, Beijing, China. LB-X, Department of Physiology, Capital University of Medical Sciences, Beijing, China.
References
Zung WWK. A rating instrument for anxiety disorders. Psychosomatics. 1971; 12: 371-379
Zung WWK, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic Further validation of the SDS. Arch Gen Psychiatry. 1965 Dec;13(6):508-15.