Effects of cranial electrotherapy stimulation on preoperative anxiety, pain and endocrine response
Lee, S-H, Kim, W-Y, Lee C-H, Min, T-J, Lee Y-S, Kim J-H. Effects of cranial electrotherapy stimulation on preoperative anxiety, pain and endocrine response. Journal of International Medical Research, 41(6) 1788–1795, 2013. Download Article.
Device
Alpha-Stim®
Key Variables
Preoperative anxiety
Objective
The purpose of this study was to investigate the efficacy of CES for the treatment of preoperative anxiety, withdrawal response during injection of rocuronium, postoperative pain, and stress hormone levels.
Design
This was an IRB approved study that used a randomized, sham controlled, double-blind design in which subjects in the treatment group received one 20 minute CES treatment the day before surgery and one 20 minute treatment just prior to surgery. The sham group received the same number and length of CES treatments with a sham CES device.
Primary Effectiveness Endpoint
The primary effectiveness endpoint was the last post-treatment scores on a 5 point Likert scale, where “1” indicated no anxiety to “5” indicated extremely anxious as compared to the sham treatment group at the endpoint of the study.
Key Inclusion Criteria
• Female participants between 20-65 years of age.
• Diagnosis of suspected thyroid cancer and scheduled for surgery.
• American Society of Anesthesiologists (ASA) physical status classification I or II.
Key Exclusion Criteria
• Pregnancy, planning to become pregnant or nursing.
• Presence of implanted pacemakers, pumps or electrical stimulators.
• Age > 65 years.
• Body mass index ≥ 25.
• Diagnosed with renal disease, endocrine or neuromuscular disease.
• Current use of psychiatric drugs.
Protocol Summary
Subjects were randomized to either an active CES or control group using computer generated random numbers. All subjects in the active and sham groups had a CES treatment between 20:00 – 22:00 PM on the day before surgery and between 7:00 – 9:00 AM on the day of surgery.
Device Application Protocol
The active CES device was pre-set at 100 µA which is a subsensory level. The sham device was identical to the active CES device, but did not emit electricity. The length of treatment, 20 minutes, was also pre-set for both active and sham devices. Anxiety was assessed in the pre-operative holding area after the CES treatment.
Study Blinding
The subjects, investigators, physicians and staff were masked as to the identity of the device, active or sham.
Pre-Specified Criteria for Success
Pre-specified criteria for success was p=0.05.
Outcome Measures
A five point Likert self-report scale was used to measure anxiety; ‘1’ indicated no anxiety, ‘2’ indicated slightly anxious, ‘3’ indicated moderate anxiety, ‘4’ indicated a lot of anxiety and ‘5’ indicated extremely anxious. A Likert scale to measure anxiety has established reliability and validity (Davey, 2007).
Results
Subjects
There were 50 subjects in this study, 25 in the active CES group and 25 in the sham CES group. All 50 subjects completed the study.
Baseline Measurements: Group Equivalence
There was no statistically significant difference at baseline between active CES and sham treatment groups on age, height or body weight.
Data Analysis
Data were analyzed using the exact V2 test, the Mann Whitney U-test and descriptive statistics.
Anxiety
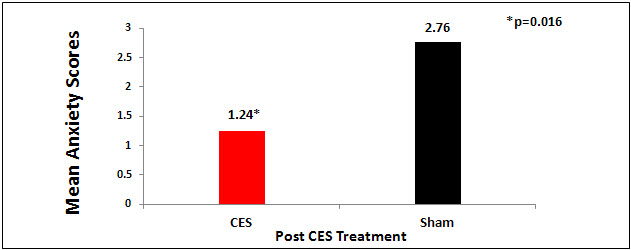
There was a significant difference between the active and sham groups for anxiety in favor of the active CES group at endpoint of study (p<0.016). The active CES group had significantly lower anxiety scores at the endpoint of the study on the Likert anxiety scale (Figure 1).
Figure 1. The CES group had significant lower anxiety scores than the sham group after CES treatment (active or sham) after CES in the preoperative holding area (p=0.016).
Quality of the Research
Strengths of the study are : a randomized, sham controlled, double-blind design was used; active and sham devices were pre-set for each group for current level and time; sham devices were the same as active devices, except they did not emit electricity. A limitation of the study is that baseline measures were not taken for anxiety prior to CES treatment. Including a baseline measure of anxiety would have strengthened this study. A limitation cited by the investigators was the inability to control for any possible placebo effect experienced by the control group due to sham treatment. They recommended that in future research, 3 groups be included; active, sham and a placebo control group. The findings of this study are consistent with the findings of other CES studies on anxiety; CES decreases anxiety.
Author Affiliations
Woon Young Kim. Professor, Department of Anesthesiology and Pain Medicine, Korea University, Ansan Hospital, Gojan-Dong, Ansan-City, Kyrungki-Do, Republic of Korea.
Reference
Davey HM, Barratt AL, Butow PN, Deeks JJ. A one-item question with a Likert or Visual Analog Scale adequately measured current anxiety. Journal of Clinical Epidemiology. 2007 Apr;60(4):356-60.