The Effect of Cranial Electrotherapy Stimulation (CES) on Pain Associated with Fibromyalgia
Cork, Randall C., Wood, Patrick, Ming, Norbert, Shepherd, Clifton, Eddy, James, Price, Larry. The Effect of Cranial Electrotherapy Stimulation (CES) on Pain Associated with Fibromyalgia. The Internet Journal of Anesthesiology, 8(2), 2004. Download Article
Device
Alpha-Stim®
Key Variable
Anxiety
Objective
To evaluate the effect of a specified treatment course with CES on fibromyalgia patients’ anxiety, pain, tender point scores, and functional impairment when compared to sham treatment under the same experimental conditions in subjects meeting the inclusion and exclusion criteria. The findings for the variable of anxiety are discussed in this abstract.
Design
An IRB approved 6 week study that included a 3 week randomized, sham treatment controlled clinical trial arm followed by a 3-week open label arm in which subjects in the sham group participated in a treatment course of CES. The investigators used the Alpha-Stim double-blind, sham-controlled RCT protocol for this study.
Primary Effectiveness Endpoint: Anxiety
The primary effectiveness endpoint for anxiety in the Phase I RCT was the change from baseline in the last post-treatment scores on the Profile of Mood States (POMS) compared to the sham treatment group at the end of week 3 of the study. The primary effectiveness endpoint for anxiety in the Phase II open label arm was the change from baseline in the last post-treatment scores on the POMS to post-test at the end of week 6 of the study.
Other effectiveness outcome measures were measured at the end of week 3 of the study and included both clinician rated and patient rated outcome measures. The following clinician rated measures was included: Tender point score evaluation. The following patient rated measures were included: McGill Pain Questionnaire (SF-MPQ), and Oswestry Score for functional impairment from pain.
Key Inclusion Criteria
• Male and female subjects with fibromyalgia ranging from 22 – 75 years of age.
• Diagnosis of fibromyalgia was verified by a physician pain specialist using the criteria of the American College of Rheumatology Criteria for the Classification of Fibromyalgia (Wolfe et al., 1990).
Key Exclusion Criteria
• Pregnancy.
• Presence of implanted pacemakers, pumps or stimulators.
• Superficial or internal ear infections.
Protocol Summary
Randomization assignment was established prior to the start of the study by the manufacturer of Alpha-Stim® CES devices. Evaluations of primary effectiveness endpoint and secondary outcome measures were taken at baseline prior to the start of the treatment period. No change was made in the medical management of the patients during the study. The protocol consisted of 2 phases:
• Phase I: 3 weeks of treatment with either the active CES device or the sham device.
Following the baseline tests, subjects were taught to use the CES device, and were instructed to use it every day for 1 hour over the 3 week period. At the end of 3 weeks, the subjects returned to the clinic, and primary and secondary outcome measures were repeated.
• Phase II: After the blinding was broken subjects in the sham group were given the option to receive active CES for 3 weeks. Those that elected to do so returned to the clinic after the 3 week period and were retested.
Device Application Summary
The active CES device was pre-set and locked by the manufacturer at 100 µA which is a subsensory level. The sham CES device was pre-set and locked by the manufacturer so that it did not emit electricity. The length of treatment, 60 minutes, was also pre-set and locked by the manufacturer for both active and sham devices. The sham device was identical in appearance to the active CES unit, but did not conduct an electrical current. The devices were randomized by the manufacturer and then packed in a device box in the order they should be given to subjects.
Study Blinding
The subjects, investigators, physicians and staff were all masked to the identity of the devices
Outcome Measures
The Profile of Mood States (POMS) was used to measure anxiety. The POMS has established reliability and validity (McNair et al., 2014).
Results
Subjects
A total of 74 subjects were enrolled, 70 females and 4 men ranging in age from 22 – 75 (M = 53) years of age. The average duration of symptoms was 7.3 years (range 1 -21 years). Subjects were randomized to the active CES group (N=39) or sham group (N=35).
Baseline Measurements
There were no statistically significant differences at baseline between active CES and sham treatment groups for any of the outcome measures.
Data Analysis
Data were analyzed with repeated measures analysis of variance, with least significant difference a posteriori testing in both Phase I and Phase II of the study.
Phase I RCT Effectiveness Results: Week 3
Seventy-four (74) subjects, 39 active CES and 35 Sham, completed the testing at the completion of week 3 visit. The active CES group had significantly decreased anxiety scores, tender points and pain compared to sham group. There was no significant difference between groups on pain as measured by the McGill Pain Questionnaire, or functional impairment. Table 1 shows results of statistical analyses of all outcome measures.
|
Outcome Variables |
Scale |
P Values |
| Anxiety | POMS |
P < 0.01 |
| Pain Intensity (NRS) | NRS |
P < 0.01 |
| Tender Point Score | Tender Point Score |
P < 0.01 |
| Pain SF-MPQ | SF-MPQ |
n.s. |
| Functional Impairment | Oswestry Score |
n.s. |
Table 1. P-Values for Comparison Between Active and Sham Treatment Means Changes
from Baseline to Week 3
Phase II Open Label Effectiveness Results: 6 Weeks
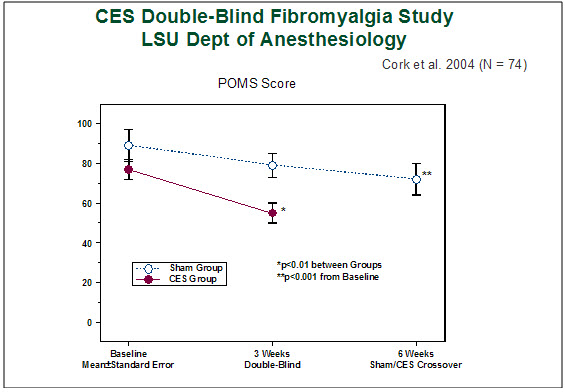
Data were analyzed with repeated measures analysis of variance, with least significant difference a posteriori testing. Table 2 shows results of statistical analyses of outcome measures at week 6. Figure 1 shows patient mood as measured by the POMS where higher scores represent more anxiety.
|
Outcome Variables |
Scale |
P Values |
| Anxiety | POMS |
P < 0.001 |
| Pain Intensity | NRS |
P < 0.001 |
| Pain | SF-MPQ |
P < 0.001 |
| Tender Point Score | Tender Point Score |
P < 0.001 |
| Functional Impairment | Oswestry Score |
n.s. |
Table 2. P-Values for Changes from Baseline within CES Open Label Group at week 6
Figure 1. Patient mood as measured by the Profile of Mood States (POMS). A higher score represents more anxiety. The anxiety reported by the CES group after 3 weeks of CES was significantly less than the anxiety reported by the sham group after 3 weeks of sham treatment (p<0.01). After crossover and 3 weeks of subsequent CES treatment, the sham group reported a significant decrease in anxiety levels from their baseline scores (p<0.001).
Quality of the Research
Strength of this study are; use of a double-blind, sham controlled RCT design; the active and sham devices were preset for time and current level, and the sham CES device was identical to the active CES device except they did not emit electricity; the study was adequately powered with an N of 74, based on the research on the effect sizes for CES for treatment of anxiety. This 2004 study measured general anxiety using the POMS scale which was commonly used at that time and has established clinical and research utility in the literature. Today, a more likely choice by investigators would be the Hamilton Anxiety Rating Scale or other similar anxiety scale. The significant finding for anxiety in this study is consistent with findings from other CES RCTs that showed CES significantly decreases anxiety.
Author Affiliation
RCC, Professor and Chair Director, Pain Management, Department of Anesthesiology
Louisiana State University School of Medicine in Shreveport, LSUHSC, LA.
References
McNair DM, Lorr M, Droppleman LF. Profile of Mood States 2nd Editon (POMS 2™). JvR Psychometrics, Accessed on 4/18/2014, http://www.psychologyafrica.com/wp-content/uploads/2013/07/Profile_of_Mood_States_2nd_Edition.pdf
Wolfe, et al. American College of Rheumatology Criteria for the Classification of Fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum, 33:160-172, 1990.